Quality Assurance Blog Posts & Updates


Inline inspection provides a modern solution, delivering accurate, real-time monitoring to ensure consistent quality control in print.
When a packaging company ships labels with incorrect barcodes or a printer delivers cartons with color variations that don’t match brand standards, the fallout goes far beyond immediate reprints.

Brandon Malz, CEO of market-leading quality inspection software, GlobalVision, gives his insights as to why document automation software is essential for success in today’s business climate.

Key Sections What is Document Automation? Why is Document Automation Important for Businesses? The “Automation Curve” Quiz: Where is Your Business…

While “going green” may be a proud point for many businesses in this day and age, in the world of packaging and design, it’s not necessarily going far enough. Instead, it’s all about being sustainable.

Document comparison software is one of those powerful tools that helps ensure the accuracy of all products that go out to market – from the inside out! Read on to explore how document comparison software [...]

Hero Spain decided to test GlobalVision’s inspection technology. The results of the inspections showed that it was very much possible to automatically proofread and inspect files with few false positives […]

RCount by GlobalVision is the market-leading automated counting system and technology that ensures compliance and traceability of sensitive materials. Your teams can now count your critical assets with 100% accuracy in record time.

For highly regulated industries like pharmaceuticals, following FDA drug labeling requirements is one of the most crucial aspects of the product lifecycle.

Verify is inspection technology reinvented—a web-based proofreading platform that ensures that you produce error-free content with the utmost ease, accuracy, and efficiency.

To avoid recalls caused by content errors, companies must inspect the artwork at every step of production, ensuring the accuracy of the text, labels, design, braille content, and barcodes.

Proofreading critical content accurately and at scale is a major concern in the Enterprise Pharma and life sciences industry though, currently, content and documentation checks are still mainly done manually.

To be successful in a consumer-driven business, it’s essential to deliver quality, innovative products, and fast! Accelerating your new product’s time-to-market can help your brand become an industry leader and stand out from the competition.

Product development and manufacturing are at the forefront of any pharmaceutical company’s business activities. Research and development, as well as product innovation help, are key factors [...]

With so much competition between brands, packaging design is a key way to distinguish your company from others and thus persuade consumers to purchase your products.
The modern business era has been characterized by a constant need to produce and deliver products with speed and efficiency. Long delays can result in financial losses and wasted resources that could have otherwise [...]

If you’ve clicked on this article, chances are you’re considering implementing a quality inspection system in your organization. Maybe your business is losing money due to delays in the quality control department [...]
From a business perspective, it makes sense to avoid waste whenever possible. Print waste in particular costs companies time and money, since any mistakes made during a print run will have to be corrected [...]

When you think about your products, what sets them apart from others in the industry? Are they more durable than the competition? Do they have better features or a more user-friendly design? Why should customers choose you over another company?

Developing pharmaceutical packaging can feel as complicated and lengthy of a process as developing the product itself. If left without logical structure, this process quickly becomes vulnerable [...]
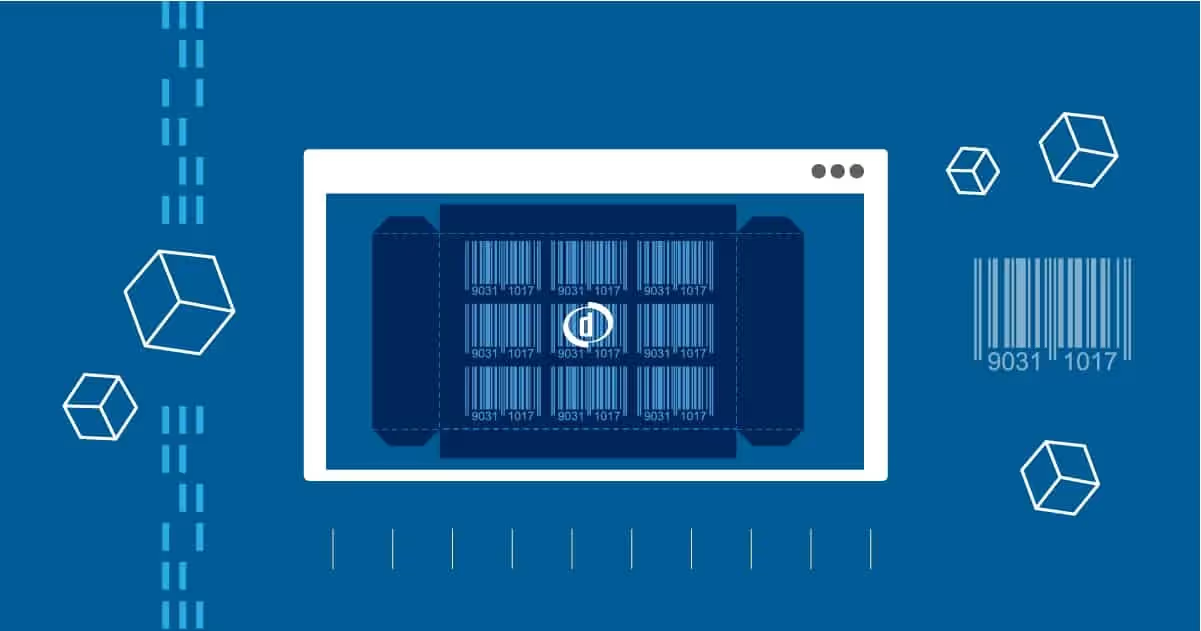
A new era of barcodes is here. With big-name retailers and consumer goods companies replacing traditional barcodes with Digimarc barcodes, printers are facing more challenges than ever when it comes to verifying […]

When you hear the word “security,” what comes to mind? Maybe you think of the stereotypical security measures that have crept into our collective consciousness as [...]

Consistency is a trademark of both validation and data integrity. In fact, without validation and the consistency for which it strives, forget data integrity. It’s like there is no data at all.

There are many ways to ensure data integrity. As automation becomes increasingly prevalent in the 21st century, software, especially on the back end, is as important as ever.

Regardless of the industry, all companies have the same goal in mind: to provide high-quality products to keep customers loyal and attract new ones. To accomplish this, each product must go through [...]

In spite of the term Software Development Lifecycle (SDLC), there aren’t concrete steps to follow when coding. Each case is somewhat different, even if there is one universal truth: Certain standards [...]

It’s easier to think of an audit trail as a collection of breadcrumbs leading out of the woods. The Bright Side of Being Audited Admittedly, the word “audit” [...]

What is a Quality Management System? A quality management system (QMS) is a term that refers to a system in charge of documenting all processes, responsibilities, and procedures for achieving quality objectives and policies.

Terms like “audit” and “inspection” are prevalent in any manufacturing business. The first one refers to analyzing manufacturing organizations and processes, whereas the second refers to any product-checking activity.

There are some constants that cannot be ignored, even between two radically different products. If you were to take those two products and examine them, there is no denying there would be a long list […]
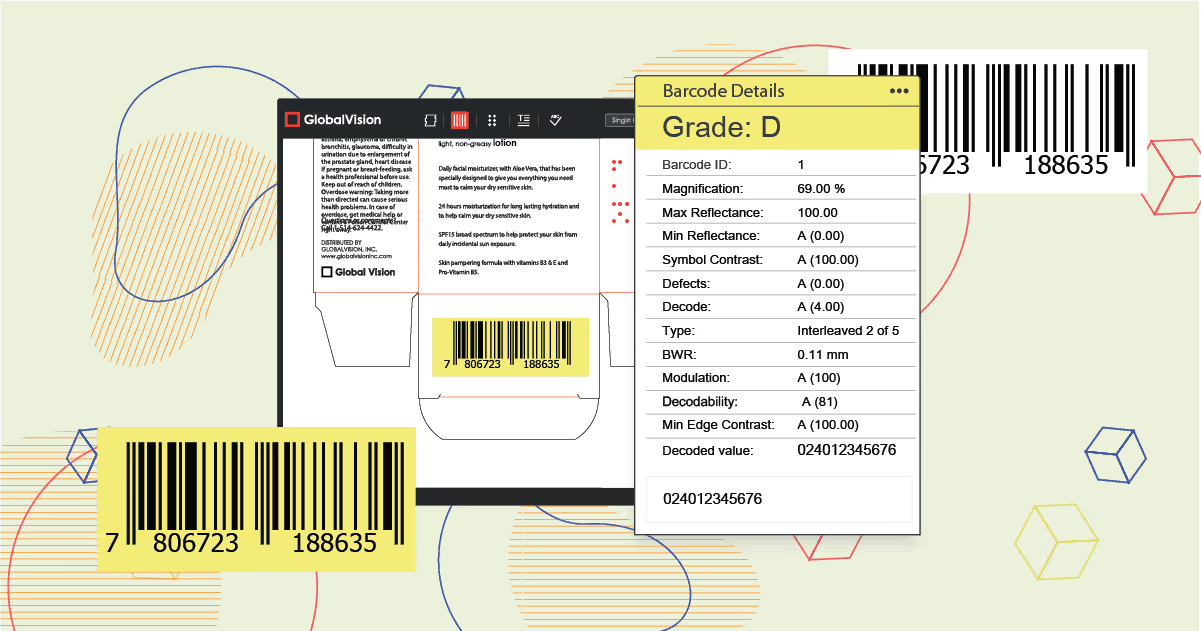
The use of barcodes dates back to the early 1960s, initially deployed within the automotive and railroad industry. In the early 1970’s they were implemented within the retail industry, mainly in grocery stores.

Business owners and manufacturers know that flawed packages carry a negative message for their brands. An imperfect package is an imperfect business, especially living in a world with customers [...]
What Does Data Integrity Mean? Data integrity refers to the fact that data must be reliable and accurate over its entire lifecycle. Data integrity and data security go hand in hand, even though they’re separate concepts.



