Compliance Updates and Blog Posts


Introduction to Pharmaceutical Artwork Management. In pharma, artwork isn’t just cosmetic. It’s how patients get the right dose, and how companies show regulators they’re following the rules.

Since the Medical Device Regulation (MDR) came into effect, medical device companies have had to rethink how they approach labeling for the EU market.

Finding the right pharmaceutical labeling company affects everything from regulatory approval timelines to patient safety outcomes.

Quality control software forms the backbone of compliance in regulated industries, yet many teams struggle with outdated tools that can't keep pace with modern packaging complexity.
Packaging accuracy directly impacts business success in regulated industries. An effective artwork management system creates measurable competitive advantages by reducing packaging errors, preventing costly recalls, and protecting brand reputation wh
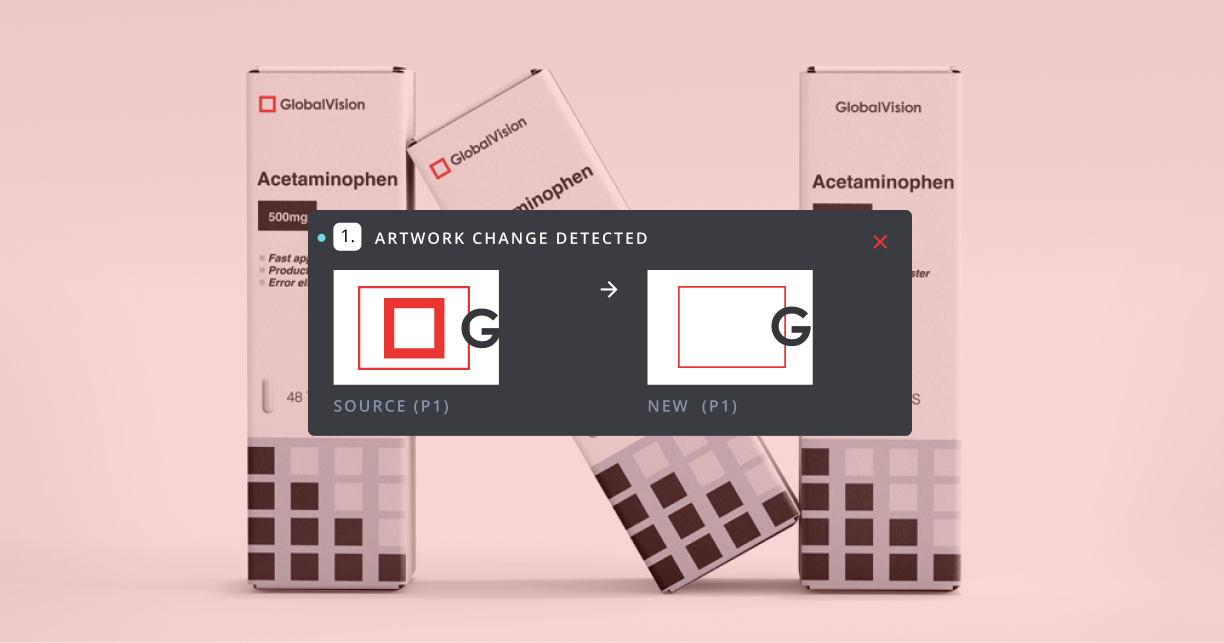
The smallest artwork error can lead to massive recalls, regulatory fines, or damaged brand reputation, especially when it appears on regulatory packaging or labeling.

In industries like pharmaceuticals, medical devices, and consumer packaged goods, getting your product labeling right is critical.

For anyone involved in the production, distribution, or regulation of pesticide products in the United States, the EPA Label Review Manual is an essential resource.

Structured Product Labeling (SPL) is the backbone of how the FDA processes and manages drug information, but it’s often overlooked. This guide breaks it down: what is SPL, why it matters, and how you can stay compliant without complicating workflows.

Explore the latest trends in e-labeling and see how life sciences companies can stay ahead by embracing digital transformation in their labeling strategies.

For life sciences companies the shift to cloud-based solutions has become crucial for managing compliance in increasingly digital workspaces.

Learn everything you need to know about compliance audits and download our checklist to take the first steps towards compliance excellence!

Providing a list of software tools that can help regulatory teams get to market faster. Intent is to help educate them on the options out there for growing their tech stack for added efficiency, and for GV to provide a perspective on tools we would

Take a deep dive into how multinational companies manage complex and evolving global health regulations, while focusing on strategies and best practices for compliance management.

An Overview of Compliance Automation The Benefits of Automation for Regulatory Compliance Where to Implement Compliance Automation? Implementing…

Why is Document Version Control Important? Use a Centralized Document Management System Implement Version…

Understanding Compliance Document Management The Importance of Compliance Document Management in Business Key Benefits of Compliance Document…

Understanding the complex regulatory compliance landscape of the European Union’s (EU) pharmaceutical industry poses a significant challenge for companies aiming to remain compliant while ensuring […]

Over the years, requirements of medicine packaging have undergone significant changes, driven by advancements in technology, changes in consumer expectations and needs, and, most importantly […]

From a quality control perspective, Braille Inspection and accuracy play an important role as Braille is mandatory on pharmaceutical packaging all across Europe, and is strongly recommended by the FDA […]

After exploring various solutions on the market, Dempsey Corporation made the decision to incorporate GlobalVision into their quality control processes. to eliminate errors and fatigue caused by manual proofreading.

As we continue to process the historic decision of the United Kingdom to exit the European Union, commonly known as Brexit, its far-reaching implications continue to unfold across various sectors.

In the digital age, where data serves as the backbone of decision-making, business operations, and technological advancements, ensuring the integrity of data has become paramount.

In today’s digital age, information security has become an utmost concern for organizations and their customers. As cyber threats continue to evolve, businesses must prioritize safeguarding […]

This comprehensive guide provides detailed insights into FDA labeling requirements and ensures your organization not only thoroughly understands labeling guidelines, but also sets best practices […]

FDA labeling requirements vary from market, product, and commodity. Each product, depending on its intention of use, has its own set of requirements that manufacturers need to follow.

To ace FDA food label compliance reviews and ensure your labels are always approved, tap into the power of automation. Automated quality control is a comprehensive solution for your compliance needs […]

In today’s digital age, a new threat presents itself to businesses that are heavily reliant on data. Many data-driven organizations rely on data integrity to be able to conduct their business operations without issues.

Now more than ever, the demand for safe and effective medical drugs is increasing on a global scale. This means that regulatory affairs teams are working overtime to interpret, apply, and communicate […]

There’s a saying: “Winning isn’t everything; It’s the only thing.” In business however, before you can win, you have to first comply with regulations.

Of note, the Food and Drug Administration (FDA) has official data integrity guidelines, out in full force as we speak. However, firms who are already following current Good Manufacturing Practices (cGMP) have nothing to worry about.

Still in its relative infancy as a discipline, regulatory affairs was created to meet a pressing need, regardless of the industry in question.

What is the FDA? The Food and Drug Administration is a government agency that operates under the United States Department of Health and Human Services.

Every company needs change, but in the midst of implementing it, only a few of them think about ISO 9000. It is mostly perceived as a purely technical standard that only specific industries have to deal with […]

One way companies can ensure high-quality standards is by demonstrating to accredited organizations that they can fulfill specific QMS requirements.

The pharmaceutical marketplace has changed dramatically over the last few years as pharmaceutical companies are moving from centralized, internal production to single-source […]



