GlobalVision to Preview ScanProof at Interphex Puerto Rico
Introducing ScanProof a major milestone in Quality Control print inspection – booth #712
Montreal, CANADA, October 09, 2014 – GlobalVision today announced that it will be offering attendees to Interphex Puerto Rico a sneak peek at its latest innovative proofreading solution, ScanProof, the world’s fastest print inspection solution for large files.
Join us booth #712 at the Puerto Rico Convention Center, San Juan, Puerto Rico, October 16-17.
Designed for printers, pharmaceutical and consumer packaged goods companies, ScanProof provides accurate quality control of all printed packaging and scanned components. ScanProof is ideal for the inspection of packaging proofs, labels, cartons and nested press sheets in both printed and PDF formats.
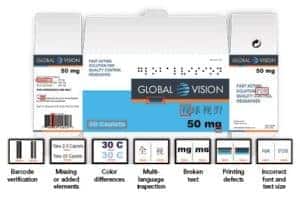
ScanProof quickly detects and identifies:
• Missing text and graphics
• Incorrect fonts and text size
• Broken type
• Color deviations
• Print defects
“ScanProof is a major milestone in Quality Control for print inspection. This unique solution is the end result of years of discovery and development working closely with our pre-media and print customers, addressing their needs for their ever changing global markets,” explained Reuben Malz, President of GlobalVision. “We are delighted to demonstrate for the first time this industry leading print inspection solution at Interphex Puerto Rico.”
ScanProof features:
• 64-bit architecture to handle large files
• Supports 1-bit TIFF files
• New PDF viewer
• Programmable layers and separation management
• System can automatically adjust and match files for overlay inspection
• Detect with pixel precision all differences regardless of sample size
• Detailed side-by-side thumbnail differences with checklist
• Multi-file processing
• Automatic repeat detection/inspection
• Barcode inspection (BarProof)
For more information or to request a demonstration of ScanProof please send an email to info@globalvisioninc.com
About GlobalVision
GlobalVision is the world leader in the design and delivery of Innovative Proofreading Technologies.
Our solutions are widely interoperable and have been integrated into the packaging workflows of leading consumer packaged goods companies, printing firms and over 72% of the major pharmaceutical industry worldwide.
GlobalVision’s complete suite of advanced solutions featuring text-based, pixel-based and Braille inspection technologies are designed to eliminate printed artwork and copy related errors, providing end-to-end security at every stage of the packaging workflow. All our proofreading solutions meet FDA 21 CFR Part 11 / EMA Annex 11 requirements.
Headquartered in Montreal, Canada, GlobalVision has worldwide representation. For more information, please visit globalvision.co.


