
Challenge: Product Lifecycle Delays Causing Financial Losses
Prior to implementing GlobalVision, Biogen struggled with certain convoluted business processes and unreliable legacy systems that caused delays in their products’ lifecycles. The processes in place made making even minor changes a difficult task.
“It was as if we had to build from the ground up every single time a change was requested.”
Richard Eyre, Associate Director of Product Lifecycle Management Regulatory CMC and Asset Development Project Management IT Systems
Their legacy systems were inefficient, resulting in regular compliance issues and data losses, hindering Biogen’s ability to maintain regulatory compliance, and reducing operational efficiency.

This in turn delayed new artwork creation, proof generation, print and packaging supplier communications, and ultimately: manufacturing lines, leading to significant financial losses worth millions.
Solution: Streamlined Artwork Development Thanks to Automated Quality Inspections
In March 2019, Biogen implemented GlobalVision’s suite of automated quality inspection tools in hopes of alleviating these critical delays. They found that the system helped them deliver artwork files exactly as intended and significantly reduced revision cycles.
Biogen was able to quantify the effectiveness of GlobalVision by compiling various success metrics including:
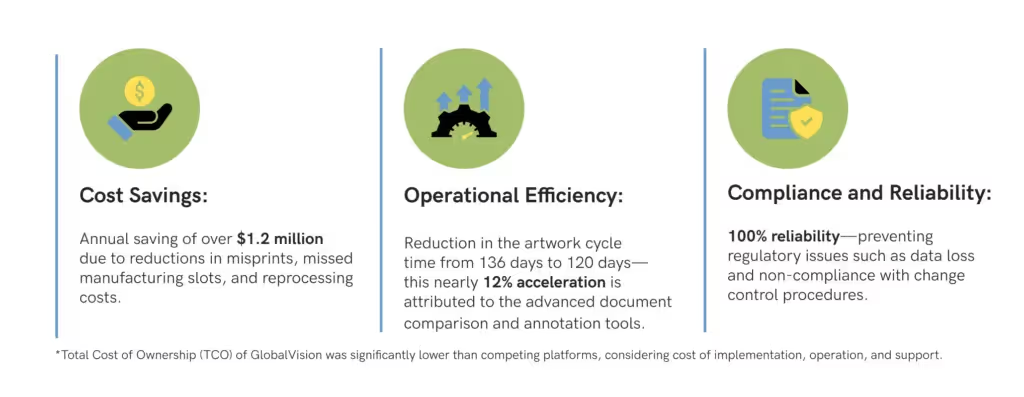
“The Artwork Design Team, Regulatory Teams, Quality Partners, and Graphic Service Providers—across all four of those functions – the time savings are associated with the move to GlobalVision.”
– Richard Eyre, Associate Director of Product Lifecycle Management Regulatory CMC and Asset Development Project Management IT Systems
Ultimately, Biogen’s implementation of GlobalVision’s market-leading software allowed them to deliver critical medicines to market faster, further solidifying the software’s position as the leading proofreading and quality inspection solution for regulated industries.The software’s capabilities such as pixel-to-pixel comparison and text comparison, quickly proved to be crucial for maintaining the integrity of product information across the product lifecycle.
“The reliability associated with both the implementation, the maintenance, and the operation of the [GlobalVision] system has been amazing and really is one of the keys to why we want to prolong the relationship with GlobalVision.”
– Richard Eyre, Associate Director of Product Lifecycle Management Regulatory CMC and Asset Development Project Management IT Systems



